usp class vi testing
USP Class VI tests and the guidelines have no alternative nonanimal methods. Many medical device companies are familiar with USP Class VI but that standard isnt as strict as ISO 10993.

Double Bagged Usp Class Vi Liquid Funnels
It was so easy in and out quickly the process was simple and the.

. Stability testing in Piscataway NJ can and should be done on many different substances and should test a variety of characteristics based on a range of specific environmental conditions. USP Class VI Certification. Cially to elastomeric closures for which the appropriate Bio-Table 1.
EMSL Analytical offers laboratory testing services sampling supplies test kits air monitoring instruments and building inspection tools to help identify mold contamination and other indoor. 49 563 reviews 309 Omni Dr Hillsborough Township NJ 08844. Developed to test drug containers the class plastics.
About USP Class VI. The species and number of animals used in this study were recommended by the USP guidelines. Open Mon 1000 am - 400 pm.
The testing for the six different class plastics levels is all done using different combinations of these three tests and different extracts. The PWTA is specific to New Jersey and it is a consumer information law that requires sellers buyers and lessors of properties with wells to test the untreated groundwater for a variety of. In fact USP Class VI is sometimes seen as a minimum.
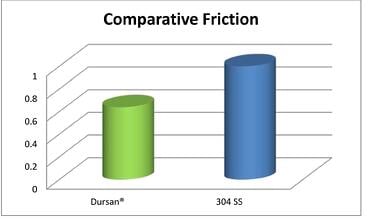
USP Class VI testing is conducted by producing an extract of the product with different extraction fluids such as polyethylene glycol and vegetable oil and injecting it in specimen rabbits and. How Dursan performed under USP Class VI test conditions. USP Class VI Plastic Tests are designed to evaluate the biological reactivity of various types of plastics materials in vivo.
Why its important for silicon CVD coatings to be USP Class VI compliant. Testing to the highest ISO-10993 standards can add months of time and be very costly according to the Medical Device Testing Guide by Toxikon Inc. USP Class VI requires the.
As our post on USP Class VI testing laid out biocompatibility is the measure of a materials lack of interaction with living tissue or a living system by not being toxic or. Intracutaneous Test are used for elastomeric materials espe-1 USP High-Density Polyethylene RS.
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

Biocompatibility Testing Of Adhesives
Watch And Learn The Benefits Of A Medical Grade Epoxy

Usp Class Plastics Pacific Biolabs

O Rings Fda And Usp Class Vi Darcoid Rubber Company Oakland California
Parker V1274 75 Usp Class Vi Biocompatibility O Ring United Seal

Rubber Fab Provides Certificate Of Compliance Documents
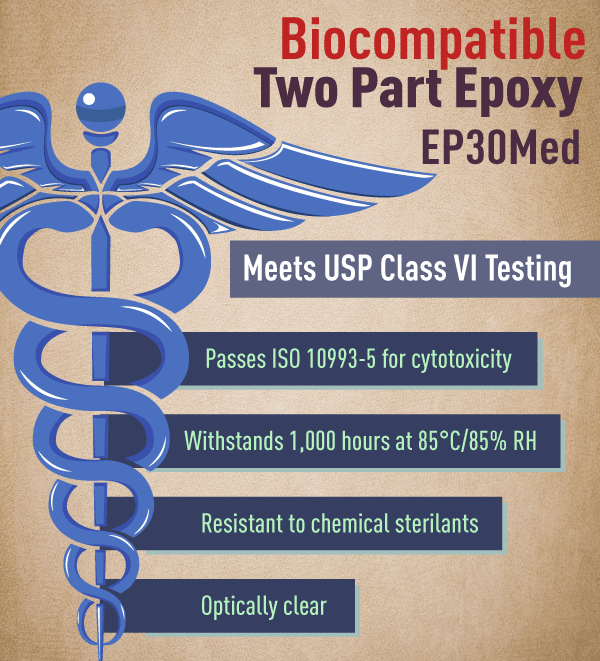
Ep30med Low Viscosity Epoxy Meets Usp Class Vi Specifications Quote Rfq Price And Buy

Usp Class Vi Certification Presco Marking Products And Engineered Films
Biopharmaceutical Usp Class Vi Gaskets Newman Sanitary Gasket

Usp Class Vi And Biocompatibility Of Products For Pharmaceutical Use Mtg
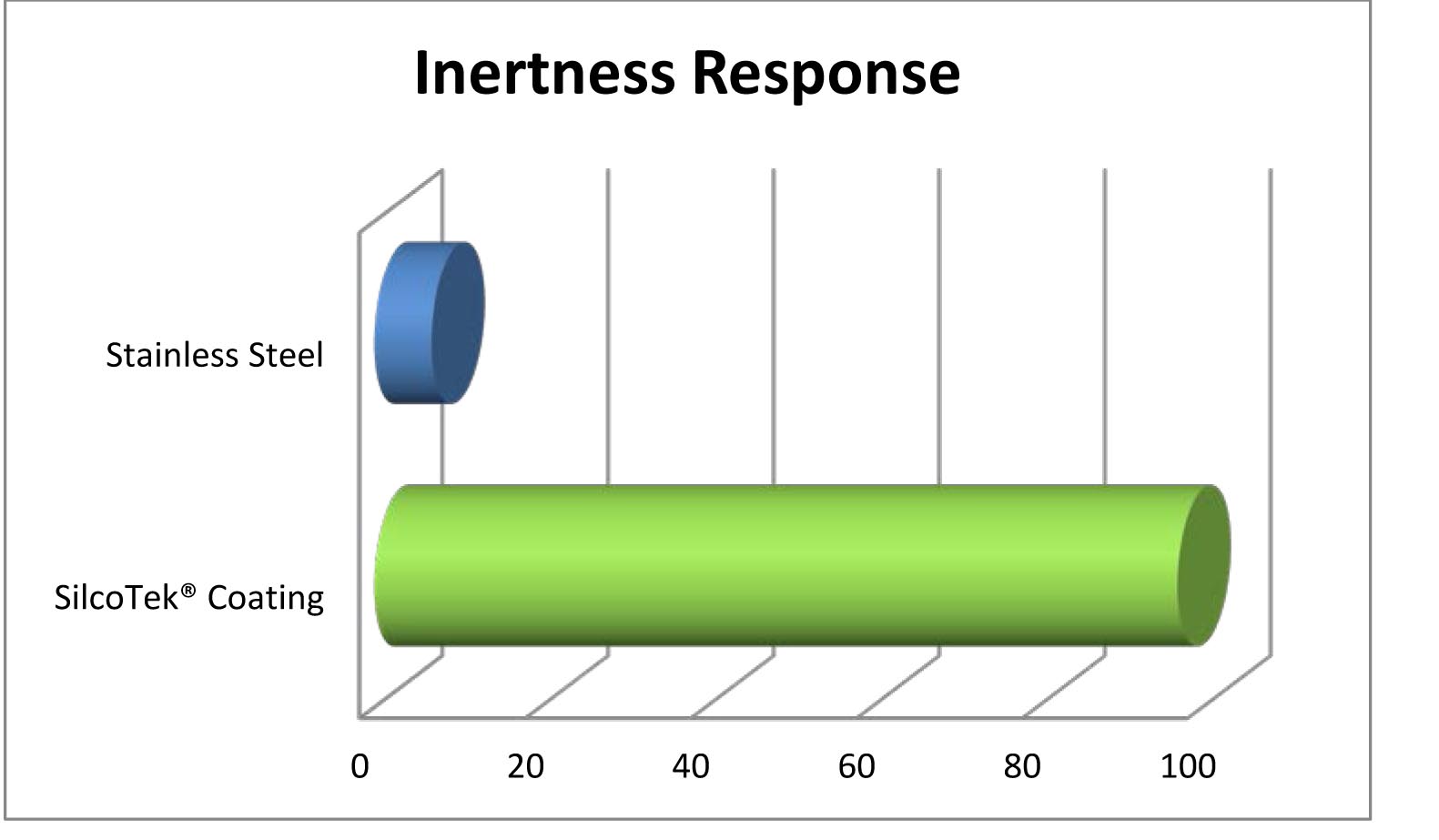
Dursan Passes Usp Class Vi Testing Why Is That Important
Dursan Passes Usp Class Vi Testing Why Is That Important

Regulatory Talks Is Usp 88 Class Vi Animal Bioreactivity Testing Necessary For Plastics Used In Manufacturing

The Value Of Usp Class Vi Testing For Medical Device Cable And Wire Medical Design Briefs


